1. 動電(dian)位極化曲線分析
不同固溶處理后的2205雙相(xiang)不(bu)銹鋼(gang)在不同溫度的3.5%NaCl溶液中的極化曲線如圖5.7所示。
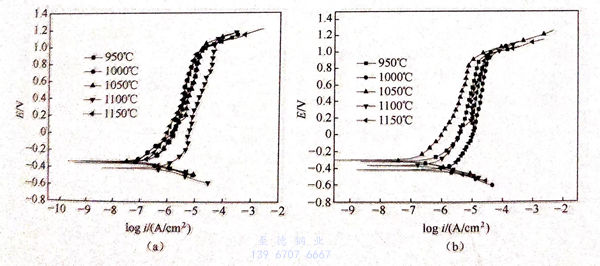
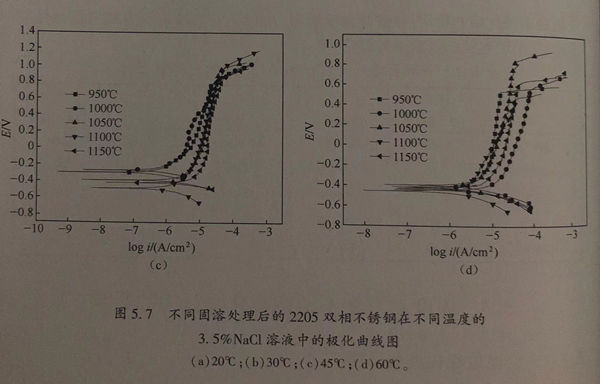
從圖5.7中可以看出,不同固溶處理的2205雙相不銹鋼在20℃、30℃、45℃的3.5%NaCI溶液中都存在一定范圍的鈍化區,且彼此的鈍化區間相差不大。但是,當3.5%NaCl溶液的溫度提升至60℃時,(950℃、1000℃、1100℃、1150℃)/30min 固溶處理的雙相不銹(xiu)鋼的鈍化范圍與1050℃/30min 固溶處理的雙相不銹鋼的鈍化范圍相比,其明顯變窄。這說明隨著溶液溫度的升高,1050℃/30min固溶處理的雙相不銹鋼的鈍化膜(mo)更加穩定。
根據GB 4334.9-1984,當腐蝕電流密度達到0.1mA/c㎡時,此時曲線上所對應的電位值就是點蝕(shi)電位。結合GB 4334.9-1984和圖5.7,得到不同固溶處理的2205雙相不銹鋼在不同溫度的3.5%NaCl溶液中的點蝕電位,如圖5.8和表5.3所示。
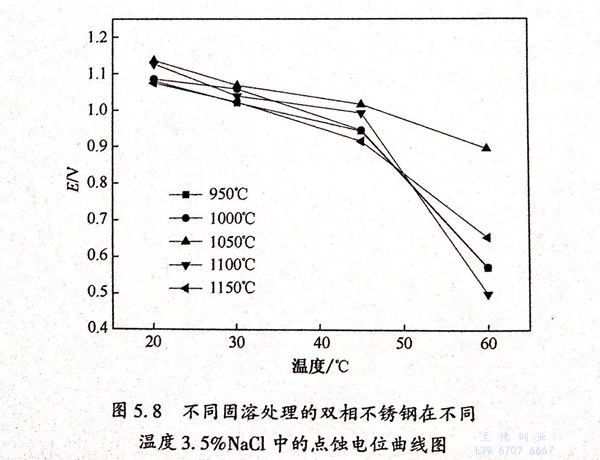
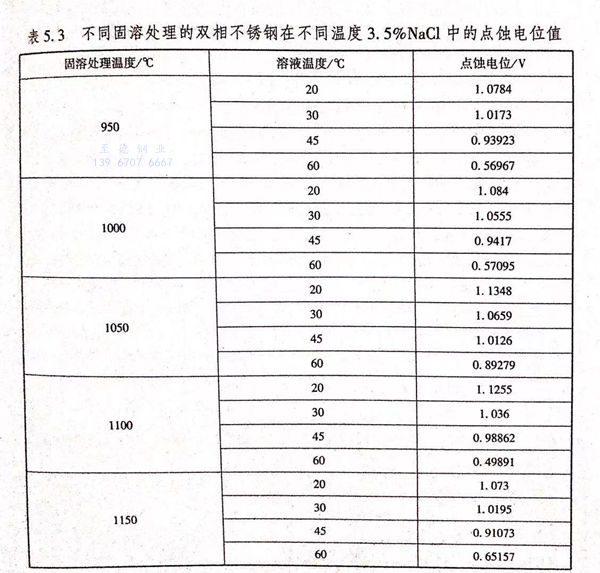
從圖5.8中可以看(kan)出,隨著3.5%NaCl溶(rong)(rong)液(ye)的(de)溫度(du)(du)(du)的(de)升高(gao),不(bu)同固(gu)(gu)(gu)溶(rong)(rong)處理的(de)雙(shuang)(shuang)相(xiang)(xiang)不(bu)銹(xiu)鋼的(de)點蝕(shi)電位(wei)(wei)(wei)下(xia)降。并(bing)且(qie)可以看(kan)出,當溫度(du)(du)(du)從20℃升高(gao)至45℃時(shi),不(bu)同固(gu)(gu)(gu)溶(rong)(rong)處理的(de)雙(shuang)(shuang)相(xiang)(xiang)不(bu)銹(xiu)鋼的(de)點蝕(shi)電位(wei)(wei)(wei)下(xia)降的(de)趨勢較(jiao)為平(ping)(ping)緩;當3.5%NaCl溶(rong)(rong)液(ye)的(de)溫度(du)(du)(du)進一步(bu)升高(gao)至60℃時(shi),(950℃、1000℃、1100℃、1150℃)/30min固(gu)(gu)(gu)溶(rong)(rong)處理的(de)雙(shuang)(shuang)相(xiang)(xiang)不(bu)銹(xiu)鋼的(de)點蝕(shi)電位(wei)(wei)(wei)急劇下(xia)降,而1050℃/30min固(gu)(gu)(gu)溶(rong)(rong)處理的(de)雙(shuang)(shuang)相(xiang)(xiang)不(bu)銹(xiu)鋼的(de)點蝕(shi)電位(wei)(wei)(wei)下(xia)降趨勢依然平(ping)(ping)緩,這說明(ming)隨著溶(rong)(rong)液(ye)溫度(du)(du)(du)的(de)升高(gao),1050℃/30min 固(gu)(gu)(gu)溶(rong)(rong)處理的(de)雙(shuang)(shuang)相(xiang)(xiang)不(bu)銹(xiu)鋼的(de)點蝕(shi)敏感性較(jiao)低,且(qie)鈍(dun)化膜更加穩定。
從(cong)(cong)(cong)表5.3中(zhong)也可(ke)以(yi)看出,對(dui)(dui)于(yu)950℃/30min固(gu)溶(rong)(rong)(rong)處(chu)(chu)理(li)(li)的(de)(de)(de)(de)雙(shuang)相(xiang)(xiang)(xiang)不(bu)(bu)(bu)銹(xiu)鋼而(er)(er)言,當3.5%NaCl溶(rong)(rong)(rong)液的(de)(de)(de)(de)溫(wen)度從(cong)(cong)(cong)20℃升(sheng)高(gao)(gao)(gao)(gao)至(zhi)(zhi)60℃時,其(qi)點(dian)(dian)蝕(shi)(shi)(shi)(shi)電(dian)(dian)位(wei)(wei)(wei)從(cong)(cong)(cong)1.0784V下(xia)(xia)(xia)(xia)降(jiang)(jiang)至(zhi)(zhi)0.56967V,降(jiang)(jiang)幅(fu)為(wei)0.50873V;對(dui)(dui)于(yu)1000℃/30min固(gu)溶(rong)(rong)(rong)處(chu)(chu)理(li)(li)的(de)(de)(de)(de)雙(shuang)相(xiang)(xiang)(xiang)不(bu)(bu)(bu)銹(xiu)鋼而(er)(er)言,當3.5%NaCl溶(rong)(rong)(rong)液的(de)(de)(de)(de)溫(wen)度從(cong)(cong)(cong)20℃升(sheng)高(gao)(gao)(gao)(gao)至(zhi)(zhi)60℃時,其(qi)點(dian)(dian)蝕(shi)(shi)(shi)(shi)電(dian)(dian)位(wei)(wei)(wei)從(cong)(cong)(cong)1.084V下(xia)(xia)(xia)(xia)降(jiang)(jiang)至(zhi)(zhi)0.57095V,降(jiang)(jiang)幅(fu)為(wei)0.51305V;對(dui)(dui)于(yu)1050℃/30min固(gu)溶(rong)(rong)(rong)處(chu)(chu)理(li)(li)的(de)(de)(de)(de)雙(shuang)相(xiang)(xiang)(xiang)不(bu)(bu)(bu)銹(xiu)鋼而(er)(er)言,當3.5%NaCI溶(rong)(rong)(rong)液的(de)(de)(de)(de)溫(wen)度從(cong)(cong)(cong)20℃升(sheng)高(gao)(gao)(gao)(gao)至(zhi)(zhi)60℃時,其(qi)點(dian)(dian)蝕(shi)(shi)(shi)(shi)電(dian)(dian)位(wei)(wei)(wei)從(cong)(cong)(cong)1.1348V下(xia)(xia)(xia)(xia)降(jiang)(jiang)至(zhi)(zhi)0.89279V,降(jiang)(jiang)幅(fu)為(wei)0.24171V;對(dui)(dui)于(yu)1100℃/30min 固(gu)溶(rong)(rong)(rong)處(chu)(chu)理(li)(li)的(de)(de)(de)(de)雙(shuang)相(xiang)(xiang)(xiang)不(bu)(bu)(bu)銹(xiu)鋼而(er)(er)言,當3.5%NaCl溶(rong)(rong)(rong)液的(de)(de)(de)(de)溫(wen)度從(cong)(cong)(cong)20℃升(sheng)高(gao)(gao)(gao)(gao)至(zhi)(zhi)60℃時,其(qi)點(dian)(dian)蝕(shi)(shi)(shi)(shi)電(dian)(dian)位(wei)(wei)(wei)從(cong)(cong)(cong)1.1255V下(xia)(xia)(xia)(xia)降(jiang)(jiang)至(zhi)(zhi)0.49891V,降(jiang)(jiang)幅(fu)為(wei)0.62659V;對(dui)(dui)于(yu)1150/30min℃固(gu)溶(rong)(rong)(rong)處(chu)(chu)理(li)(li)的(de)(de)(de)(de)雙(shuang)相(xiang)(xiang)(xiang)不(bu)(bu)(bu)銹(xiu)鋼而(er)(er)言,當3.5%NaCl溶(rong)(rong)(rong)液的(de)(de)(de)(de)溫(wen)度從(cong)(cong)(cong)20℃升(sheng)高(gao)(gao)(gao)(gao)至(zhi)(zhi)60℃時,其(qi)點(dian)(dian)蝕(shi)(shi)(shi)(shi)電(dian)(dian)位(wei)(wei)(wei)從(cong)(cong)(cong)1.073V下(xia)(xia)(xia)(xia)降(jiang)(jiang)至(zhi)(zhi)0.65157V,降(jiang)(jiang)幅(fu)為(wei)0.42143V.綜上所(suo)述(shu)可(ke)以(yi)看出,1050℃/30min固(gu)溶(rong)(rong)(rong)處(chu)(chu)理(li)(li)的(de)(de)(de)(de)雙(shuang)相(xiang)(xiang)(xiang)不(bu)(bu)(bu)銹(xiu)鋼的(de)(de)(de)(de)點(dian)(dian)蝕(shi)(shi)(shi)(shi)電(dian)(dian)位(wei)(wei)(wei)較(jiao)高(gao)(gao)(gao)(gao),以(yi)及點(dian)(dian)蝕(shi)(shi)(shi)(shi)電(dian)(dian)位(wei)(wei)(wei)隨著溶(rong)(rong)(rong)液溫(wen)度升(sheng)高(gao)(gao)(gao)(gao)而(er)(er)下(xia)(xia)(xia)(xia)降(jiang)(jiang)的(de)(de)(de)(de)幅(fu)值(zhi)較(jiao)低(di),說明其(qi)1050℃固(gu)溶(rong)(rong)(rong)處(chu)(chu)理(li)(li)的(de)(de)(de)(de)雙(shuang)相(xiang)(xiang)(xiang)不(bu)(bu)(bu)銹(xiu)鋼的(de)(de)(de)(de)點(dian)(dian)蝕(shi)(shi)(shi)(shi)敏感(gan)性較(jiao)低(di)。而(er)(er)950℃/30min、1000℃/30min、1100℃/30min、1150℃/30min 固(gu)溶(rong)(rong)(rong)處(chu)(chu)理(li)(li)的(de)(de)(de)(de)不(bu)(bu)(bu)銹(xiu)鋼的(de)(de)(de)(de)點(dian)(dian)蝕(shi)(shi)(shi)(shi)電(dian)(dian)位(wei)(wei)(wei)都比1050℃/30min固(gu)溶(rong)(rong)(rong)處(chu)(chu)理(li)(li)的(de)(de)(de)(de)不(bu)(bu)(bu)銹(xiu)鋼的(de)(de)(de)(de)點(dian)(dian)蝕(shi)(shi)(shi)(shi)電(dian)(dian)位(wei)(wei)(wei)低(di),耐點(dian)(dian)蝕(shi)(shi)(shi)(shi)性能有所(suo)下(xia)(xia)(xia)(xia)降(jiang)(jiang)。
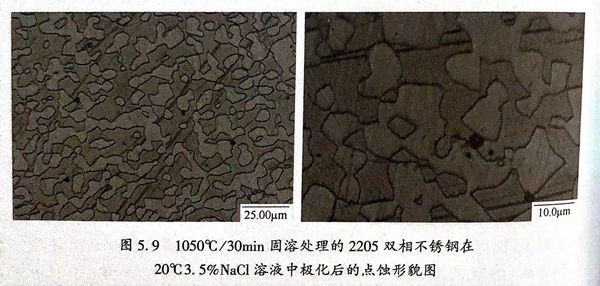
1050℃/30min固溶(rong)處理的(de)2205雙(shuang)相不銹鋼在20℃3.5%NaCl溶(rong)液中極化后的(de)點蝕形貌如圖(tu)5.9所示。圖(tu)中淡(dan)色部(bu)分為奧(ao)氏體,深(shen)色部(bu)分為鐵素(su)體,黑色部(bu)分為點蝕坑。
從圖5.9中可以看,點蝕易發生于鐵素體和鐵素體-奧氏體晶界處,并且點蝕易向鐵素體中發展。在雙相不銹鋼中,Cr、Mo、N是主要的耐點蝕元素,鐵素體含有更多量的Cr和Mo;而奧氏體還有更多的Ni和Mn,并且N元素富集于奧氏體相中,提高局部腐蝕抗力。雙相不銹鋼的耐點蝕當量值可由“PREN=%Cr+3.3×%Mo+16×%N”計算得到,耐點蝕當量值越高,雙相不銹鋼的耐點蝕能力越強。隨著固溶處理的溫度的升高,鐵素體的含量逐漸增加,而奧氏體的含量不斷減少,造成鐵素體中的Cr、Mo被稀釋,導致鐵素體的耐點蝕當量逐漸降低;而隨著固溶處理溫度的升高,奧氏體的含量降低,造成奧氏體中的N濃度升高,奧氏體耐點蝕當量逐漸升高。
雙(shuang)相(xiang)不(bu)銹(xiu)鋼中(zhong)(zhong)含(han)(han)(han)有較高含(han)(han)(han)量(liang)(liang)的(de)(de)(de)(de)(de)Cr和Mo,在(zai)氧化(hua)性(xing)(xing)介質中(zhong)(zhong)其表面會生成(cheng)一層鈍(dun)化(hua)膜保護基(ji)(ji)體(ti)(ti)(ti)。由于CI-對(dui)鈍(dun)化(hua)膜存(cun)(cun)(cun)在(zai)破壞性(xing)(xing),甚至通(tong)過鈍(dun)化(hua)膜的(de)(de)(de)(de)(de)間隙,與基(ji)(ji)體(ti)(ti)(ti)金屬接觸,使(shi)得(de)基(ji)(ji)體(ti)(ti)(ti)發生溶(rong)(rong)解。鈍(dun)化(hua)膜的(de)(de)(de)(de)(de)穩定性(xing)(xing)能(neng)(neng)(neng)(neng)夠反映(ying)其對(dui)金屬的(de)(de)(de)(de)(de)保護程度(du),而(er)(er)(er)點(dian)(dian)(dian)(dian)蝕(shi)電(dian)位(wei)能(neng)(neng)(neng)(neng)夠反映(ying)鈍(dun)化(hua)膜的(de)(de)(de)(de)(de)穩定性(xing)(xing)。通(tong)常情況下(xia),點(dian)(dian)(dian)(dian)蝕(shi)電(dian)位(wei)越高,金屬的(de)(de)(de)(de)(de)耐點(dian)(dian)(dian)(dian)蝕(shi)性(xing)(xing)能(neng)(neng)(neng)(neng)越好。由第3章可(ke)知,當(dang)固溶(rong)(rong)處理(li)(li)(li)的(de)(de)(de)(de)(de)溫(wen)度(du)為1050℃時,2205雙(shuang)相(xiang)不(bu)銹(xiu)鋼基(ji)(ji)體(ti)(ti)(ti)中(zhong)(zhong)的(de)(de)(de)(de)(de)鐵素(su)體(ti)(ti)(ti)的(de)(de)(de)(de)(de)含(han)(han)(han)量(liang)(liang)與奧(ao)(ao)(ao)氏(shi)體(ti)(ti)(ti)的(de)(de)(de)(de)(de)含(han)(han)(han)量(liang)(liang)之比約為1:1,且鐵素(su)體(ti)(ti)(ti)和奧(ao)(ao)(ao)氏(shi)體(ti)(ti)(ti)的(de)(de)(de)(de)(de)分(fen)(fen)(fen)布(bu)較均(jun)勻,Cr和Mo在(zai)鐵素(su)體(ti)(ti)(ti)中(zhong)(zhong)的(de)(de)(de)(de)(de)含(han)(han)(han)量(liang)(liang)分(fen)(fen)(fen)布(bu)和N在(zai)奧(ao)(ao)(ao)氏(shi)體(ti)(ti)(ti)中(zhong)(zhong)的(de)(de)(de)(de)(de)含(han)(han)(han)量(liang)(liang)分(fen)(fen)(fen)布(bu)較均(jun)勻,整體(ti)(ti)(ti)的(de)(de)(de)(de)(de)耐點(dian)(dian)(dian)(dian)蝕(shi)當(dang)量(liang)(liang)較高,表現出(chu)(chu)較好的(de)(de)(de)(de)(de)耐點(dian)(dian)(dian)(dian)蝕(shi)性(xing)(xing)能(neng)(neng)(neng)(neng)。當(dang)固溶(rong)(rong)處理(li)(li)(li)的(de)(de)(de)(de)(de)溫(wen)度(du)為950℃時,大量(liang)(liang)的(de)(de)(de)(de)(de)σ相(xiang)會沿著鐵素(su)體(ti)(ti)(ti)-奧(ao)(ao)(ao)氏(shi)體(ti)(ti)(ti)晶界析出(chu)(chu),而(er)(er)(er)σ相(xiang)是一種硬脆相(xiang),其周圍會存(cun)(cun)(cun)在(zai)貧(pin)鉻區,它的(de)(de)(de)(de)(de)存(cun)(cun)(cun)在(zai)顯著降(jiang)低(di)材料(liao)的(de)(de)(de)(de)(de)力學性(xing)(xing)能(neng)(neng)(neng)(neng)和耐蝕(shi)性(xing)(xing)能(neng)(neng)(neng)(neng),且σ相(xiang)的(de)(de)(de)(de)(de)析出(chu)(chu)使(shi)其存(cun)(cun)(cun)在(zai)區域的(de)(de)(de)(de)(de)鈍(dun)化(hua)膜薄弱,使(shi)得(de)點(dian)(dian)(dian)(dian)蝕(shi)電(dian)位(wei)較低(di),點(dian)(dian)(dian)(dian)蝕(shi)更容易發生。當(dang)固溶(rong)(rong)處理(li)(li)(li)的(de)(de)(de)(de)(de)溫(wen)度(du)升高至1150℃時,基(ji)(ji)體(ti)(ti)(ti)中(zhong)(zhong)的(de)(de)(de)(de)(de)鐵素(su)體(ti)(ti)(ti)的(de)(de)(de)(de)(de)含(han)(han)(han)量(liang)(liang)百(bai)(bai)分(fen)(fen)(fen)比為59%,而(er)(er)(er)奧(ao)(ao)(ao)氏(shi)體(ti)(ti)(ti)的(de)(de)(de)(de)(de)含(han)(han)(han)量(liang)(liang)百(bai)(bai)分(fen)(fen)(fen)比為41%,鐵素(su)體(ti)(ti)(ti)含(han)(han)(han)量(liang)(liang)過多,導致鐵素(su)體(ti)(ti)(ti)的(de)(de)(de)(de)(de)耐點(dian)(dian)(dian)(dian)蝕(shi)當(dang)量(liang)(liang)下(xia)降(jiang),造(zao)成(cheng)耐點(dian)(dian)(dian)(dian)蝕(shi)性(xing)(xing)能(neng)(neng)(neng)(neng)下(xia)降(jiang)。
2. 交流(liu)阻(zu)抗測試分析
不同固溶處理的2205雙相不銹鋼在不同溫度的3.5%NaCl溶液中的Nyquist圖如圖5.10所示。從圖5.10中可以看出,在不同溫度的3.5%NaCl溶液中的不同固溶處理的雙相不銹鋼的Nyquist圖中的高頻和低頻處,都存在一個容抗弧,說明雙相不銹鋼表面存在一層鈍化膜。所以該電化學過程中,存在兩個時間常數。并且,曹楚南的電化學阻抗譜分析也認為,不銹鋼鈍化過程存在兩個時間常數,這與本實驗所測數據是一致的。而本實驗的電化學阻抗測試是在雙相不銹鋼自鈍化狀態下進行的,而雙相不銹鋼在自腐蝕電位下形成的表面鈍化膜是存在缺陷的,材料表面由于缺陷的存在而暴露于電解質溶液中,所以采用如圖5.11所示的等效電路(其中,R1為溶液電阻;R2為電荷轉移電阻;R3為鈍化膜電阻;Cdl為雙電層電容;Cf為鈍化膜膜電容)。
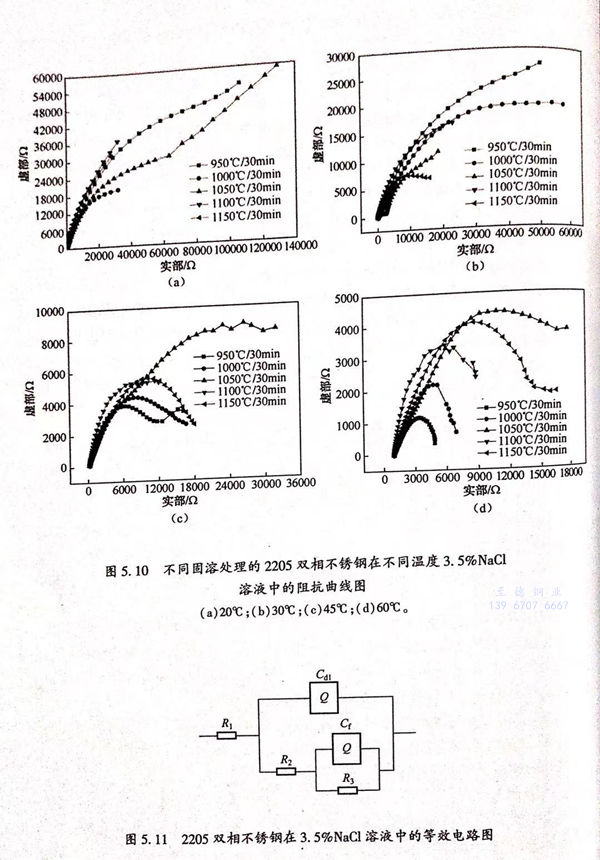
根據圖(tu)5.11的(de)等(deng)效電路,利(li)用軟件ZSimpWin進(jin)行(xing)阻(zu)(zu)抗(kang)的(de)擬合,得到如(ru)圖(tu)5.12所(suo)示的(de)電荷(he)轉(zhuan)移(yi)(yi)電阻(zu)(zu)曲(qu)線(xian)圖(tu)和(he)如(ru)圖(tu)5.13所(suo)示的(de)鈍(dun)化(hua)膜阻(zu)(zu)抗(kang)值(zhi)曲(qu)線(xian)圖(tu)。電荷(he)轉(zhuan)移(yi)(yi)電阻(zu)(zu)阻(zu)(zu)抗(kang)值(zhi)和(he)鈍(dun)化(hua)膜阻(zu)(zu)抗(kang)值(zhi)如(ru)表5.4所(suo)列。
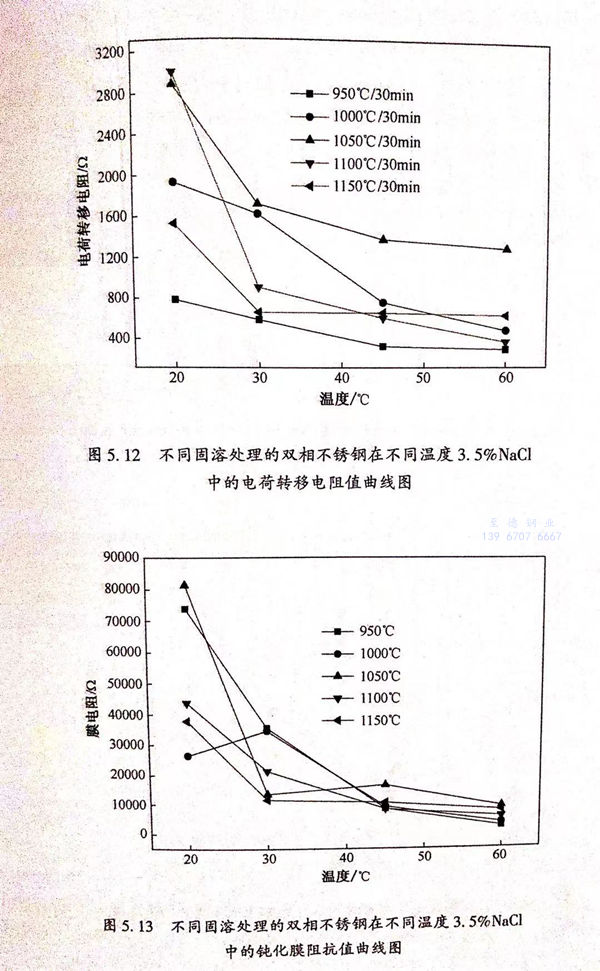
從(cong)圖5.12中可以看出,隨著3.5%NaCl溶液溫度(du)的升高,不(bu)同固溶處(chu)理的雙(shuang)相不(bu)銹鋼的電(dian)(dian)(dian)化(hua)學反(fan)(fan)應的電(dian)(dian)(dian)荷(he)轉移電(dian)(dian)(dian)阻降低,電(dian)(dian)(dian)化(hua)學反(fan)(fan)應的阻力下降,電(dian)(dian)(dian)化(hua)學反(fan)(fan)應變快。耐蝕(shi)性能下降。
從圖5.13中可以看出,隨著3.5%NaCl溶液溫度的升高,不同固溶處理的雙相不銹鋼的鈍化膜阻抗值下降,鈍化膜穩定性變差,雙相不銹鋼的耐點蝕性能下降。一方面,O2在溶液中的溶解度隨著溫度的升高而降低,當NaCI溶液的溫度升高時,溶液中含氧量降低,導致雙相不銹鋼表面鈍化膜形成所需的O元素下降,降低鈍化膜形成的可能性;另一方面,隨著溶液溫度的升高,鈍化膜的溶解速度升高,導致雙相不銹鋼的耐點蝕性能下降。
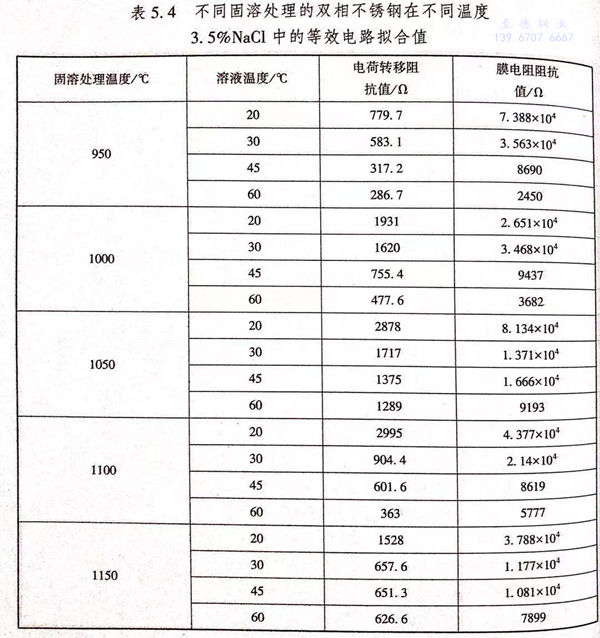
從(cong)表5.4中可以看出;不銹鋼的(de)(de)電(dian)(dian)化學(xue)反應(ying)的(de)(de)電(dian)(dian)荷轉(zhuan)移電(dian)(dian)阻阻抗(kang)值遠小(xiao)于不銹鋼的(de)(de)鈍化膜阻抗(kang)值,說(shuo)明(ming)雙相不銹鋼在0.5mol/L 3.5%NaCl溶液中的(de)(de)耐蝕(shi)性主要(yao)是(shi)由(you)其表面的(de)(de)鈍化膜的(de)(de)穩定性決定。
從圖(tu)5.12和(he)圖(tu)5.13中可(ke)以(yi)看(kan)出,1050℃/30min固(gu)溶(rong)處(chu)理的(de)(de)(de)(de)(de)雙(shuang)相(xiang)(xiang)不銹鋼的(de)(de)(de)(de)(de)鈍化(hua)膜(mo)(mo)阻(zu)抗值和(he)電荷(he)(he)轉移電阻(zu)阻(zu)抗值較高(gao),說明(ming)1050℃/30min固(gu)溶(rong)處(chu)理的(de)(de)(de)(de)(de)試(shi)(shi)樣的(de)(de)(de)(de)(de)鈍化(hua)膜(mo)(mo)較穩定(ding),電化(hua)學反應阻(zu)力較高(gao),腐(fu)(fu)蝕(shi)速率(lv)較慢,耐(nai)蝕(shi)性(xing)(xing)能較好。而950℃固(gu)溶(rong)處(chu)理的(de)(de)(de)(de)(de)試(shi)(shi)樣中存在(zai)較多σ相(xiang)(xiang),降(jiang)低(di)了表(biao)面(mian)的(de)(de)(de)(de)(de)鈍化(hua)膜(mo)(mo)的(de)(de)(de)(de)(de)穩定(ding)性(xing)(xing),表(biao)現出較低(di)的(de)(de)(de)(de)(de)鈍化(hua)膜(mo)(mo)阻(zu)抗值;同(tong)時在(zai)其周圍存在(zai)貧鉻區,加速了腐(fu)(fu)蝕(shi),表(biao)現出較低(di)的(de)(de)(de)(de)(de)電荷(he)(he)轉移電阻(zu)值。對(dui)于1150℃/30min 固(gu)溶(rong)處(chu)理的(de)(de)(de)(de)(de)試(shi)(shi)樣,其組(zu)織中含有過量(liang)的(de)(de)(de)(de)(de)鐵(tie)素體,導致耐(nai)點(dian)蝕(shi)當量(liang)降(jiang)低(di),點(dian)蝕(shi)電位較1050℃/30min固(gu)溶(rong)處(chu)理的(de)(de)(de)(de)(de)雙(shuang)相(xiang)(xiang)不銹鋼的(de)(de)(de)(de)(de)點(dian)蝕(shi)電位低(di)。
以(yi)上結(jie)果(guo)表明,阻抗(kang)測(ce)試(shi)結(jie)果(guo)與(yu)極化(hua)曲(qu)線測(ce)試(shi)得到的結(jie)果(guo)是一致的,二者都說明1050℃/30min固溶處理的試(shi)樣(yang)的耐點蝕(shi)性能較好(hao)。



